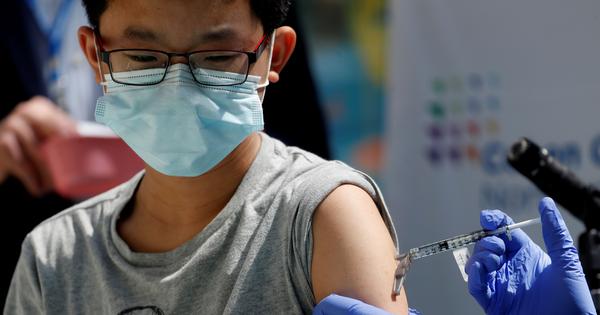
The European Medicines Agency on Friday approved the coronavirus vaccine developed by pharmaceutical company Moderna for children aged 12 to 17 years. The vaccine is already authorised for use in the European Union among beneficiaries aged 18 and above.
The medicine regulator said that a study conducted among 3,732 children in the 12-17 age group showed that Moderna’s Spikevax jab produced a similar antibody response as seen in young adults aged 18 to 25 years.
The most common side effects of the Spikevax vaccine in children were similar to those in adults, the European Medicines Agency said. The side effects include pain and swelling at the injection site, headache, nausea, vomiting and fever.
The Moderna vaccine now awaits formal approval from the European Commission, following which it would be allowed to roll out the shots for teenagers in European Union countries. The European Commission typically follows recommendations from the medicine regulator.
In May, Moderna…