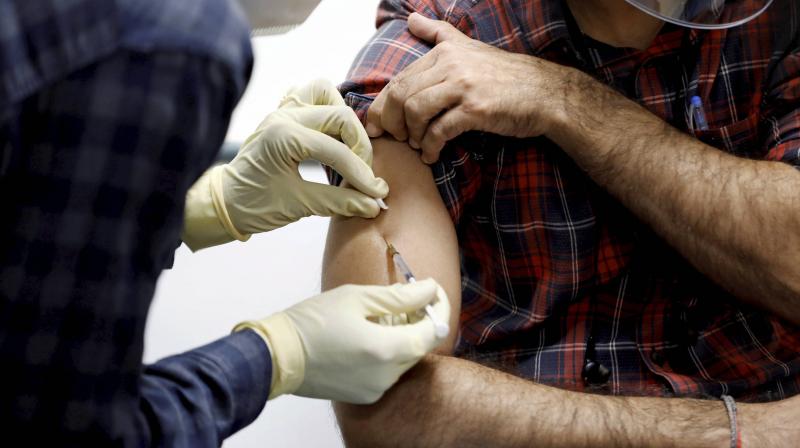
India’s first indigenous vaccine against COVID-19, Covaxin, showed enhanced immune response without any serious side effects in the participants enrolled for the phase 1 trials, according to the results published in The Lancet Infectious Disease journal.
Developed by Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV), Pune, the vaccine has been granted emergency use authorisation in clinical trial mode’ by the Indian government.
Covaxin, which is now undergoing phase-3 trials, had raised concerns among experts over its emergency approval earlier this month by India’s drug regulator.
The vaccine, codenamed BBV152, was well tolerated in all dose groups with no vaccine-related serious adverse events, noted the authors of the study funded by Bharat Biotech.
The same results were earlier published in the preprint server medRxiv in December.
However, there has been no new data released in the…