India
pti-Madhuri Adnal
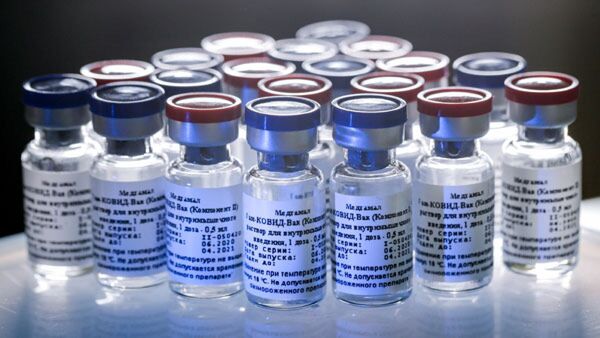
New Delhi, Dec 01: Dr Reddy’s Laboratories and Russian Direct Investment Fund (RDIF) on Tuesday said they have commenced adaptive phase 2/3 clinical trials for the Sputnik V COVID-19 vaccine in India. The trials have commenced after receiving the necessary clearance from the Kasauli-based Central Drugs Laboratory, they added.

This will be a multicentre and randomised controlled study, which will include safety and immunogenicity study, the Hyderabad-based drug maker and RDIF said in a joint statement.
The clinical trials are being conducted by JSS Medical Research as the clinical research partner. Further, Dr Reddy’s has partnered with the Biotechnology Industry Research Assistance Council (BIRAC), Department of Biotechnology (DBT) for advisory support and to use BIRAC’s clinical trial centres for the vaccine, it added.
Recently, RDIF had announced the second interim analysis of…